Tuberculosis, alongside HIV/AIDS and malaria, is counted as one of the three major infectious diseases. The use of a breakthrough drug developed by a Japanese pharmaceutical company is making its way across the world as part of the quest to conquer the illness, which threatens global health.
Tuberculosis (TB), which spread profusely in the countries of Western Europe during the industrial revolution, was once a threat to the entire world. However, from the 19th century onwards, improvements in living standards and healthcare led to a decline in the number of persons afflicted with the disease. Now, TB is considered by people in those countries to be a disease of the past. That assumption, however, is incorrect. According to a report by the World Health Organization (WHO), an estimated 1.7 billion people are currently infected with the bacteria that causes TB worldwide. Even now, 1.5 million people die from the disease each year, making it one of the top 10 causes of death in the world. In fact, outbreaks sporadically occur not only in developing countries, but in Western countries as well.
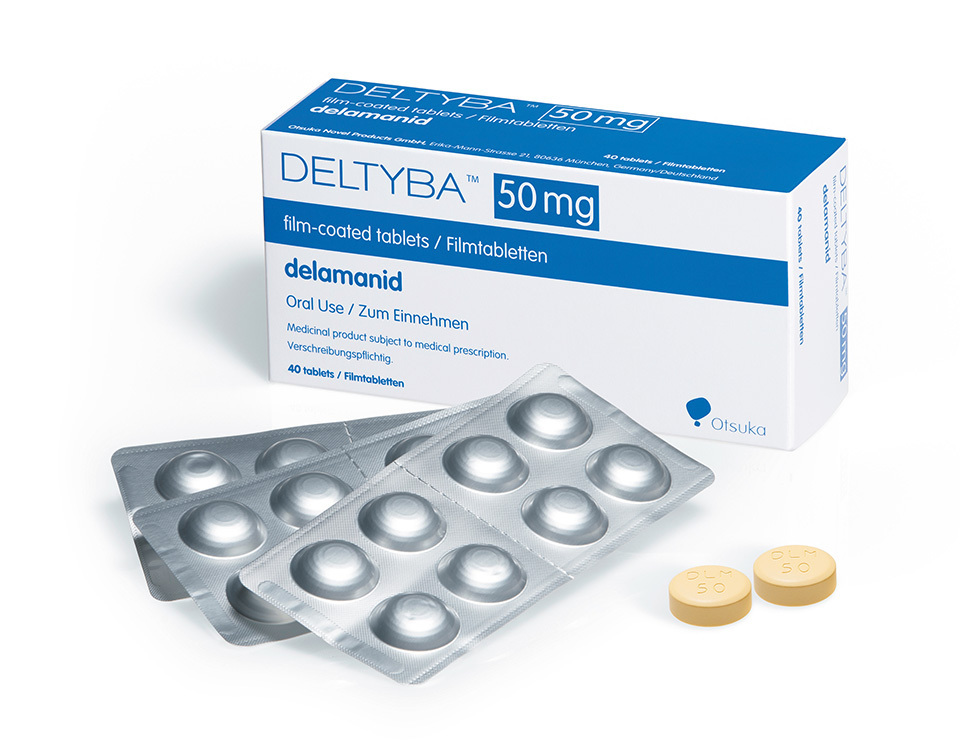
Exacerbating the problem in recent years has been the appearance of multidrug-resistant tuberculosis (MDR-TB) strains that show resistance to existing TB drugs. At the Kyushu-Okinawa Summit in 2000, Japan made combating infectious diseases, including TB, one of the summit’s major agenda topics. Driven by Japanese initiatives to tackle infectious diseases, the Global Fund to Fight AIDS, Tuberculosis, and Malaria was formed in 2002. Amid renewed awareness of TB as a global health problem, an anti-TB drug developed by a Japanese pharmaceutical company is offering new hope. The drug in question is delamanid, which was developed by Otsuka Pharmaceutical Co., Ltd., and received regulatory approval in the EU and Japan in 2014. As the first new anti-TB drug in about 40 years, it is proving to be effective against resistant strains and its use is increasing throughout the world.
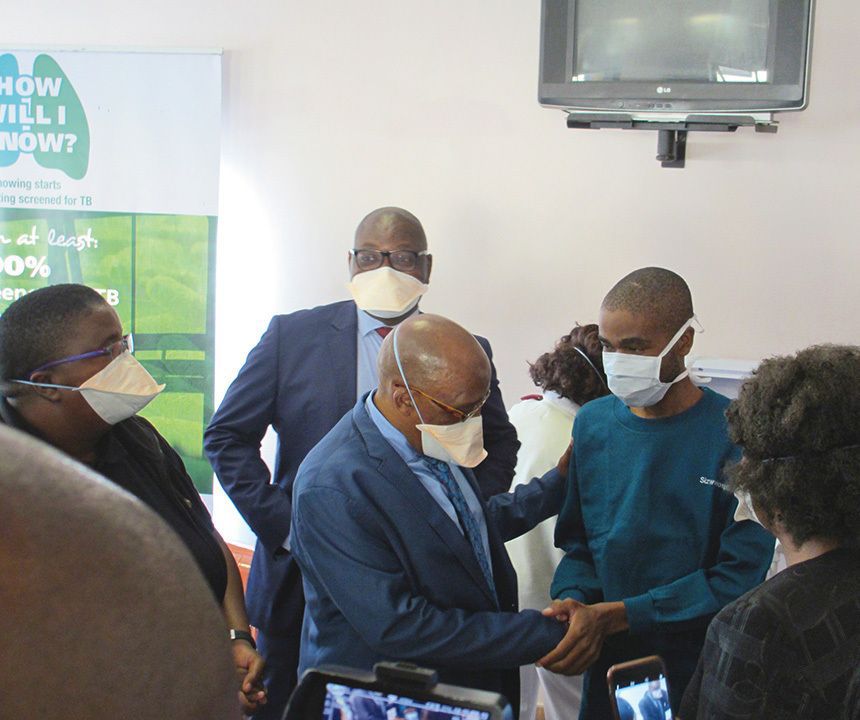
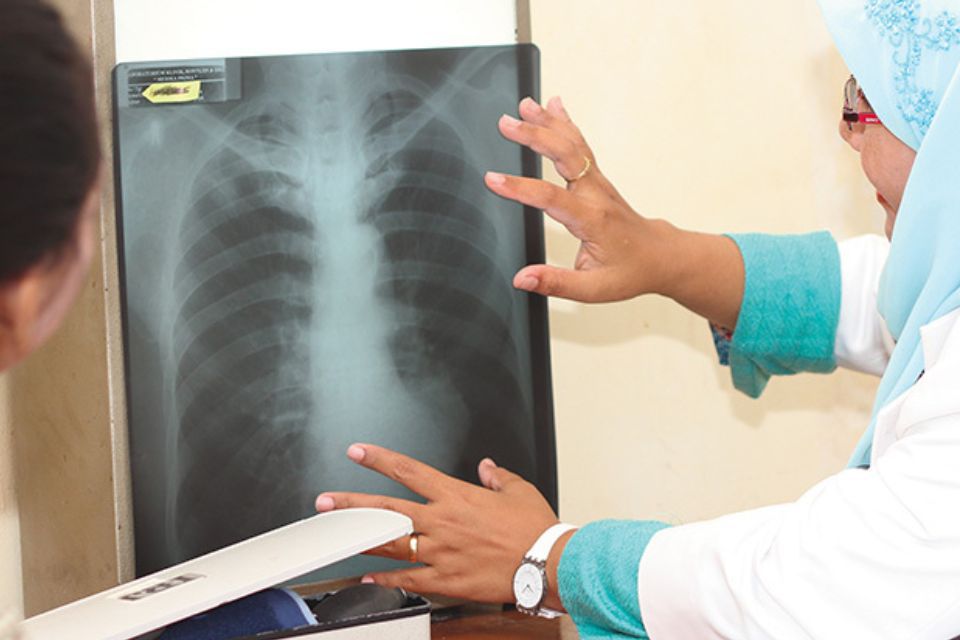
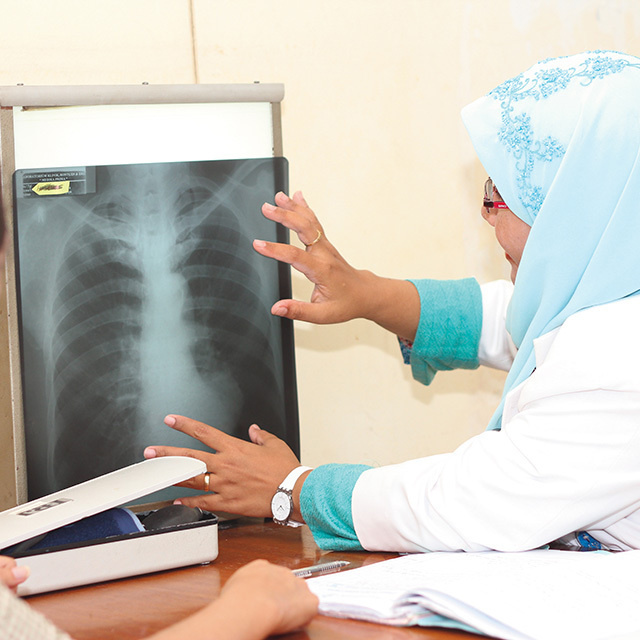
On World TB Day, March 24, 2017, Otsuka Pharmaceutical, in cooperation with South Africa’s Ministry of Health and a non-governmental organization, launched an access program for delamanid (trade name Deltyba) that is still in progress. The image shows one of the first patients to have received the drug.
When a standard treatment for TB was established in the 1970s, the development of anti-TB drugs came to a standstill around the world, but Otsuka Pharmaceutical continued its development. “Patients must receive TB treatment over a much longer time compared with other infectious diseases. In quite a few cases, treatment is halted because the side effects are too severe, and without administering continuous treatment, its elimination becomes difficult. We believed that this was a therapeutic field that showed the promise of improvement from a quality-of-life perspective as well,” says KAWASAKI Masanori, Global TB Project leader. “If no one else was going to do it, we felt that we must continue the research, and that ultimately led to the development of delamanid.”
Rather than being administered by injection or drip, delamanid is taken orally. That is a huge advantage because oral medicine is much easier for patients to manage versus injectable drugs. Another benefit of delamanid is that there are few drug interactions with the treatments for HIV/AIDS or diabetes, meaning it could also be effective for patients with coexisting medical conditions.
After earning its first approval in Europe in 2014, delamanid has since been approved in 15 countries, including South Africa and India. In the Russian Federation, the major pharmaceutical company R-Pharm was granted licensing rights in 2017 as part of the Eight-Point Cooperation Plan. Furthermore, as of the end of 2019, the use of delamanid is now possible in over 100 countries, thanks to an agreement in 2016 between Otsuka Pharmaceutical and the Stop TB Partnership, an organization established in collaboration with WHO and other partners.
The development of a drug to defeat bacteria that cause TB strains, which are difficult for antibiotics to act on, was not easy. The development team at Otsuka Pharmaceutical was exhaustively selective when deciding which candidate compounds to choose as the starting point of the drug discovery process. Through repeated trial and error, such as adopting unorthodox drug synthesis methods, the team finally achieved its goal.
Presently, the team is developing a next-generation drug candidate that will work by a different mechanism of action from delamanid. In February 2020, Otsuka Pharmaceutical joined hands with the Bill & Melinda Gates Foundation, Gates Medical Research Institute, Johnson & Johnson, Evotec SE, and GlaxoSmithKline plc, to set up the “PAN-TB collaboration,” a consortium established to accelerate new treatments for TB on a global scale. The company is providing its knowledge gained so far through TB research. Assuring us that his team will continue to direct their efforts toward fighting TB, Kawasaki says, “As demonstrated by the novel coronavirus crisis, it is important that all countries cooperate in treating infectious diseases as a global issue. As part of that, our mission is to create an environment where treatment can be administered to patients easily and more effectively.”

When Otsuka Pharmaceutical began drug discovery in 1971, it selected TB as one of its first research themes. Its untiring research efforts yielded fruit, realizing innovation that has spurred new drug development.

The Global TB Project, driven by Otsuka Pharmaceutical, is comprised of members from various countries (photo taken at the Princeton office in the United States).